What Best Describes the Action of Sodium Bicarbonate
NAC may be administered orally for which of the following conditions. Which of the following best describes the action of sodium bicarbonate.

Sodium Bicarbonate An Overview Sciencedirect Topics
Alkalinization of urine 3.

. Sodium bicarbonate will increase the level or effect of flecainide by passive renal tubular reabsorption - basic urine. Aspirin cyclic antidepressants. Sodium bicarbonate will decrease the level or effect of fosamprenavir by increasing gastric pH.
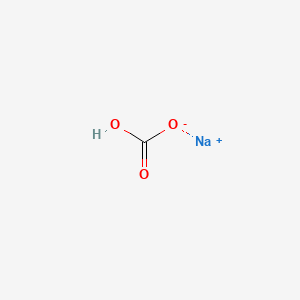
Sodium bicarbonate is a chemical compound with the formula NaHCO3. - increases blood pH. Buffers metabolic acidosis and lactic acid buildup in the body caused by anaerobic metabolism secondary to severe hypoxia by reacting with hydrogen ions to form water and carbon dioxide.
So something as simple as baking soda can often give almost instant relief for a wide range of medical situations. 1 mmolml of Na HCO. Sodium Na is the principal cation of the extracellular fluid and it maintains fluid and electrolyte disturbances.
Correction of profound metabolic acidosis especially that complicating cardiac arrest 2. Action Sodium bicarbonate reacts with hydrogen ions to form water and carbon dioxide and thereby can act to buffer metabolic acidosis By increasing the plasma concentration of bicarbonate blood pH rises. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise.
Urinary and serum alkalinization to enhance the elimination of drugs by ion trapping and minimize drug distribution respectively. - Prolonged cardiac arrest after adequate ventilation. IE- Mechanism of Action.
Sodium bicarbonate in water dissociates to sodium Na and bicarbonate HCO3 - ions. - Acts as a buffering agent. Treatment of drug overdoses whereby the offending agent has sodium channel blocking properties.
Mechanism of Action of Sodium Bicarbonate. Sodium bicarbonate is a systemic alkalizer which increases plasma bicarbonate buffers excess hydrogen ion concentration and raises blood pH thereby reversing the clinical manifestations of acidosis. Sodium Na is the principal cation of extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances.
How does Sodium Bicarbonate work on the body. Saliva facilitates swallowing by making the food bolus slippery. Bicarbonate HCO 3- is a normal constituent of body fluids and the normal plasma level ranges from 24 to 31 mmol mEqliter.
Sodium bicarbonate in water dissociates to provide sodium Na and bicarbonate HCO 3- ions. The onset of action occurs within 30 minutes and the effect lasts for 1 to 2 hours. 2021-1-14 Describe the pharmacology of sodium bicarbonate.
Sodium bicarbonates ability to neutralize acid helps treat conditions related to high acidity in bodily fluids such as indigestion which is caused by too much acid in the stomach. The alkaline properties of sodium bicarbonate help in an effective way in the treatment of heartburn acid and digestive indigestion many of the components of the diet contain meat dairy products sugars alcohol and beverages which contain caffeine which can produce a lot of acids in the body Sodium bicarbonate can be used as an antacid by mixing a. Bicarbonate HC03 - is a normal constituent of body fluids.
The main reason to use sodium bicarbonate is to help buffer the lactic acid produced when the lactic acid energy system is utilized true Testosterone has been shown to increase strength even without resistance training. Sodium bicarbonate neutralizes the acid bolus. Chymotrypsin and trypsin break down proteins into amino acids.
The various uses and proposed mechanisms of action of sodium bicarbonate include. CO2 levels in the blood which is increased by intake of sodium bicarbonate is one vital key to oxygen delivery to the cells. 1 Aspirin overdose 2 Acetaminophen overdose 3 Before cardiac angiography to prevent renal failure 4 Barbiturate overdose a.
Its also how you can make sodium carbonate another useful chemical also called washing soda. Applies only to oral form of both agents. The resultant effect restores intracellular potassium levels to normal without decreasing total body potassium stores.
INDICATIONS FIELD USE. A Facilitate swallowing b Stomach muscle contraction c Neutralize the acid bolus d Breakdown of proteins. Circulatory overload and hypernatremia can occur when large volumes of hypertonic sodium bicarbonate are given.
Clear colourless - 1264284 in an aqueous solution. Antacid Chemical An inorganic salt Presentation Tablets. Gastrin causes stomach muscle contraction.

Sodium Bicarbonate An Overview Sciencedirect Topics

No comments for "What Best Describes the Action of Sodium Bicarbonate"
Post a Comment